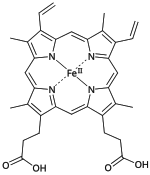
Heme B
| |

| |
| Names | |
|---|---|
| Other names
Iron protoporphyrin IX,
protoheme IX | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.114.904 |
| MeSH | Heme+b |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C34H32O4N4Fe | |
| Molar mass | 616.487 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Heme B or haem B (also known as protoheme IX) is the most abundant heme.[1] Hemoglobin and myoglobin are examples of oxygen transport proteins that contain heme B. The peroxidase family of enzymes also contain heme B. The COX-1 and COX-2 enzymes (cyclooxygenase) of recent fame, also contain heme B at one of two active sites.
Generally, heme B is attached to the surrounding protein matrix (known as the apoprotein) through a single coordination bond between the heme iron and an amino-acid side-chain.
Both hemoglobin and myoglobin have a coordination bond to an evolutionarily-conserved histidine, while nitric oxide synthase and cytochrome P450 have a coordination bond to an evolutionarily-conserved cysteine bound to the iron center of heme B.
Since the iron in heme B containing proteins is bound to the four nitrogens of the porphyrin (forming a plane) and a single electron donating atom of the protein, the iron is often in a pentacoordinate state. When oxygen or the toxic carbon monoxide is bound the iron becomes hexacoordinated. The correct structures of heme B and heme S were first elucidated by German chemist Hans Fischer.[2]
References
[edit]- ^ Ogun, Aminat S.; Joy, Neena V.; Valentine, Menogh (2022), "Biochemistry, Heme Synthesis", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30726014, retrieved 2023-01-03
- ^ Fischer, H.; Orth, H. (1934). Die Chemie des Pyrrols. Liepzig: Akademische Verlagsgesellschaft.